





Remember the periodic table? There are over 100 elements on it.
As scientists we like to put things into categories, so we’ve broken the periodic table up into 2 categories.
Metals and Non-

Metals and non-

As you can see from above, we have split the periodic table into metals and non-
Metals and non-
Metals are very good conductors of electricity. This mean electricity can travel quickly through it.
Metals are also good conductors of heat. They heat up quickly, glow and cool quickly.
While non-
When a rocket re-
First the appearance of metals compared to non-
Metals are often shiny, while non-

Usually most metals are in a solid state at room temperature (except mercury), while roughly half the non-
Metals are denser. This means that if you a cube of carbon, while the same dimensions as a cube of iron. The iron will weigh more.
Metals are much stronger than non-
They are also malleable. This means they can bend without breaking, while non-
So under pressure, a metal will bend much more than a non-
When you hit it, does it make a ringing noise? If yes then you’ve got a metal. Metals are called Sonorous because they make a ringing sound when you hit them.
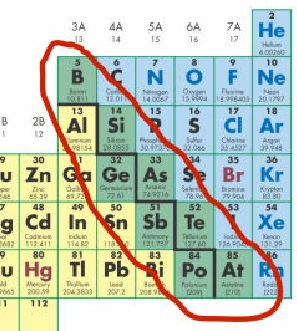
The problem is in life, not everything fits into a nice little category. Some elements near the stepped line are called metalloids.
Metalloids are elements which have properties of both metals and non-
Boron (B), silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), tellurium (Te), polonium (Po) and astatine (At)
Questions
1. Which of the following properties belong to most metals?
-
-
2. Which metal is the exception to the melting point rule of most metals?
3. Metals are often magnetic, research and find where this might come in handy.
4. Explain how a mercury filled thermometer works
5. As you know, not all metals are equal. Why might iron man abandon his old iron suit design and create a new one using titanium?





Gallium is a metal, which is solid at room temperature, but at only 30 degrees it becomes a liquid.
Notice how the spoon melts in warm water (to the left)
