Hydrochloric Acid -

Making
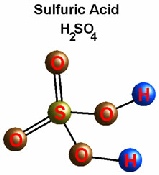

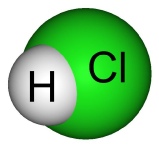
They all contain Hydrogen
So, why is that important.
To create salt, we can add an acid with a metal element or a compound.
The hydrogen atom of the acid is replaced by atoms of the metal element.
We’ve all seen salt before. We know what it looks like, we even know what it tastes like. The salt that you talk about is probably Sodium chloride.
However, did you know that there are different types of salts
Sodium Chloride Copper Chloride Sodium sulfate Zinc Sulfate




So how do we make a salt then?
Simple. We mix a metal element with an acid.
Look at the 3 acids below. What do they have in common?
Look at the animation to the left. Lets add Zinc (Zn) to Sulphuric acid (H2SO4)
The Zinc kicks off the Hydrogen and attaches to the sulphur and oxygen molecules, creating Zinc Sulphate (ZnSO4) and Hydrogen(H2).
Try these questions out
The first 2 are done for you
a. Zinc + Sulphuric acid = Zinc Sulphate + Hydrogen
b. Sodium + hydrochloric acid = Sodium Chloride + Hydrogen
c. Magnesium + hydrochloric acid = _________ ________ + _________
d. Copper + hydrochloric acid = _________ _______ + ____________
e. Sodium + sulphuric acid = ___________ _________ + __________
f. Zinc + hydrochloric acid = _________ __________ + __________
g. Copper + sulphuric acid = ___________ _________ + _________
So, as you can see from above, every time we add a metal with an acid it creates a salt and hydrogen gas.
But that’s not the only way we can make a salt.
Reacting an acid with a base can also make salts. However, unlike a metal and acid producing salt and hydrogen. A base and acid produce salt and water (H2O).
Sodium hydroxide + Hydrochloric acid = Sodium Chloride + water
Copper oxide + nitric acid = Copper nitrate + water
One thing you may have noticed from the equations above is that
Hydrochloric acid is a chloride maker
Sulphuric acid is a sulfate maker
Nitric acid is a nitrate maker
Try the questions below
Question 1.
a. Sodium Hydroxide + Hydrochloric acid = Sodium Chloride + water
b. Copper oxide + Hydrochloric acid = ________ _________ + ______
c. Sodium hydroxide + Sulphuric acid = _______ ________ + ________
d. Zinc oxide + Sulphuric acid = __________ _________ + ___________
e. Zinc oxide + Hydrochloric acid __________ ________ + ____________
f. Copper oxide + Sulphuric acid = ___________ ________ + __________
g. Sodium hydroxide + Nitric acid = ____________ __________ + _________
2. Write out the chemical formula for:
Sodium hydroxide
hydrochloric acid
Water
Nitric acid
Magnesium
Zinc.
(the chemical formula for Sulphuric acid is H2SO4)
3. Draw the following molecules:
a. Water
b. Hydrochloric acid
c. Sodium hydroxide