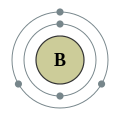
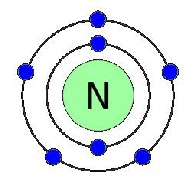
Groups are the vertical columns. The elements in a group have a similar properties.
Their Melting point, Boiling point and density are similar to each other.
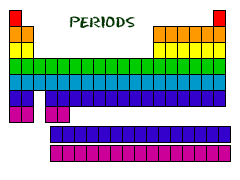
Periods are the horizontal rows. Again we can number the main group 1-7.
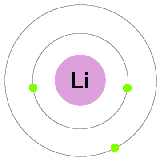
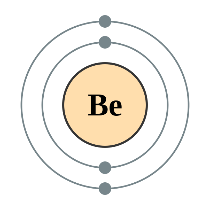
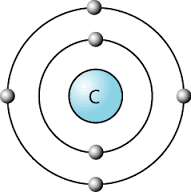
All the elements in the rows have the same number of atomic orbitals. This means they have the same number of shells as the element next to them.
If we look at the noble gases, what we see is, as we move down, the atoms gain an extra ring (another outer shell).
At the outer most shell, all the elements of group have the same amount of electrons. We call this, their Valence electrons.
Questions
1. List 4 properties that are different between metals and non-metals
2. Diamonds are very shiny and tough. Are they a metal or a non-metal, explain your answer.
3. List 1 group on the periodic table (just use the elemental symbol)
4. List one period on the periodic table (just use the elemental symbol)
5. What do elements of the same group have in common?
6. If we could see down to the atomic level, what would the elements Potassium (K), Calcium (Ca), Titanium (Ti) and Manganese (Mn) have in common?
7. Draw a Helium atom and state the groups name it belongs too.
8. A brand new element has been found. This element will sit just bellow Francium (Fr) on the periodic table and have an atomic number of 119. This new element is called Razinium (Rz).
Using your knowledge of metals and non-metals, groups and periods answer the questions bellow
i. How many valence electrons will a razinium atom have
ii. Is this a metal or non-metal
iii. Based on your answer in 8ii will razinium be a good conductor of electricity?
iiii. How will Razinium react with water?