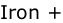Questions
1. Explain what is meant by the term Displacement and give one example
2. What is a Diatomic molecule and give one example
3. The sentence bellow has 5 errors in it. Re-write the sentence with the corrections and underline them.
In a displacement reaction, a less reactive metal pushes out a more reactive metal from its compound. For example, iron displaces aluminium from aluminium oxide
4. Using a real life example, draw a cartoon strip to illustrate displacement. (Similar to the example in the title).
5. Fill in the following blanks
a. Zinc + Copper sulphate → _______ Sulphate + _______
b. Iron + Zinc Chloride → __________ Chloride + _______
c. Aluminium + Copper Oxide → ________ Oxide + ________
d. Potassium + Lithium Oxide → _______ ______ + ______
e. Zinc + Lead Oxide → _______ ______ + ______
f. Copper + Silver nitrate → _______ ______ + ______
g. Calcium + Aluminium Sulphate → _______ ______ + ______
h. Calcium + Iron Oxide → _______ ______ + ______
i. Potassium + _______ Oxide → ______ _____ + Aluminium
j. Lead + Lithium Chloride → ________ _______ + _______
k. Calcium + Sodium Chloride → ________ _______ + _____
l. ______ + Magnesium Oxide → Lithium Oxide + Magnesium
m. Zinc + ______ Chloride → ______ ______ +Lead
n. ________ + ______ ______ → Copper oxide + Silver
o. _______ + Sodium ______ → Potassium Sulphate + _______
p. _______ + ______ _______ → Iron Chloride + Iron
q. ______ + _____ _____ → Zinc nitrate + Lead
6. Balance the following equations
a. Al + Fe2O3 → Al2O3 + Fe
b. Zn + Fe2O3 → Zn2O3 + Fe
c. Mg + Fe2O3 → Mg2O3 + Fe
d. Fe + CuO → Fe2O3 + Cu
e. Li + CuO → Li2O3 + Cu
7. Fill in the blanks
a. Zinc + Copper Sulphate → _______ Sulphate + _______
__ + CuSO4 → ZnSO4 + __
b. Potassium + Lithium Oxide → _______ ______ + ______
___ + Li2O → ___ + ___
c. Calcium + Sodium Chloride → ________ _______ + _____
____ + NaCl → CaCl2 + _____
d. __________ + Iron Oxide → Aluminium _____ + ______
Al + ____ → ______ + Fe
e. Silver + ______ _____ → Lead Sulphate + ______
___ + PbSO4 → ___ + Ag